2022 Abstract Submission
Abstract Presentation Information: If you did not receive additional details of your platform or poster presentation on June 13, please check your junk mail box and then contact us at meetings@cancergenomics.org.
2022 CGC Annual Meeting Abstracts
Many thanks to all those who submitted abstracts for CGC 2022.
We received 146 abstracts, a record number of submissions for the CGC.
See below to know where CGC 2022 abstracts originated...
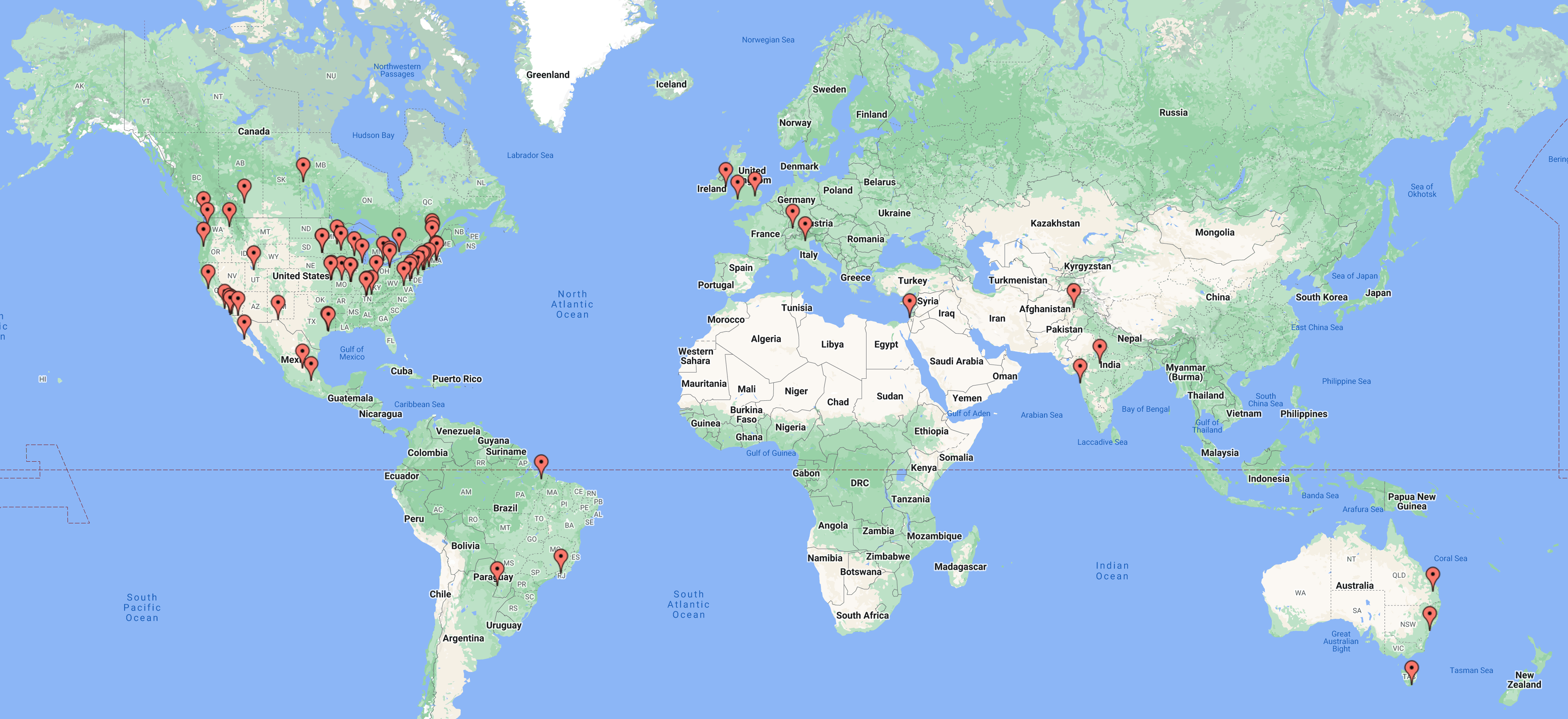
We received 146 abstracts, a record number of submissions for the CGC.
See below to know where CGC 2022 abstracts originated...

Abstract Submission:
- Abstracts should represent original scientific research.
- Abstract submission is for both Oncology and Constitutional topics. Suggested topics are found below. Abstracts will be considered outside these topics.
- There is a 250 word limit on the text in the body of submitted abstracts.
- Accepted abstracts will be designated as poster or virtual platform presentations.
- All accepted abstracts will be published in Cancer Genetics.
- Abstracts must be submitted by Monday, February 28, 2022, 11:59 PM (midnight) Pacific Standard Time.
- Abstract acceptance notifications are expected to be sent on or before April 8, 2022.
- Presenting authors of accepted abstracts must register and pay for meeting attendance by April 25, 2022 to secure abstract publication in Cancer Genetics.
- While in the submission module, if you need to return to a previous page in the submission process, please use the back arrow on your browser.
- Abstracts are selected based on scientific rigor.
- Abstracts commercial in nature will not be accepted. Companies who choose to support the meeting have other opportunities to present data about specific products.
- If you have challenges with your submission, please email meetings@cancergenomics.org.
2022 Abstract Submission Topics:
- Bioinformatics and Artificial Intelligence
- Challenges and Approaches in Reporting Results
- Electronic Health Records and Genomic Data
- Integration of Genomic Results in the EMR, Instead of Reporting as PDF or Text File Format
- Genomic Resources for Variant Curation and Standardization
- Cytogenomic Testing in the Era of Targeted Therapy
- Technical Topics (e.g., Assay Validation, Mosaicism, Emerging Technologies, Liquid Biopsy, etc.)
- Solid Tumors (Testing Algorithms, Cases, and/or Clinical Management)
- Immunotherapy and Tumor Mutational Burden
- Hematologic Malignancies (Testing Algorithms, Cases, and/or Clinical Management)
- Constitutional Disorders (Testing Algorithms, Cases, and/or Clinical Management)
- Hereditary Cancer Syndromes / Cancer Predisposition
- Vascular Anomalies and Tissue Overgrowth Syndromes
- Incidental Findings of Clinical Significance During Genomic Testing
- Pharmacogenomics (Testing Algorithms, Cases, and/or Clinical Management)
- Equity (Disparities) in Genomic Testing
- Regulatory and Ethical Consideration in Genomic Testing
- Guidelines and Regulatory Compliance
- Economic Affairs (e.g., Billing, Coding, Reimbursement)
- Social and Ethical Considerations
- Other Oncology and Constitutional Topics
Please do not hesitate to submit an abstract that does not fit into a category above.
Abstract Presentation Information:
- If you did not receive additional details of your platform or poster presentation on June 13, please check your junk mail box and then contact us at meetings@cancergenomics.org.

